Zantac Lawsuits: Carcinogen Prompts Recall
ALERT: April 1, 2020 – “The U.S. Food and Drug Administration today announced it is requesting manufacturers withdraw all prescription and over-the-counter (OTC) ranitidine (Zantac) drugs from the market immediately.”
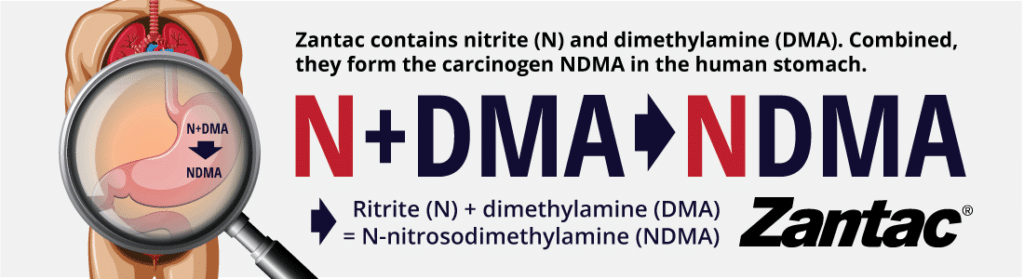
Since the 1980s, ranitidine (brand name: Zantac) has been associated with NDMA. While Zantac is not contaminated with NDMA, it contains nitrite (N) and dimethylamine (DMA). Combined, they form the carcinogen NDMA.
The Food and Drug Administration (FDA) was notified in June of 2019 that routine testing done in Valisure’s labs had linked Zantac to N-nitrosodimethylamine (NDMA). FDA’s own testing found unacceptable levels of NDMA in Zantac.
“The results [of several case–control studies and one cohort study] are supportive of the assumption that NDMA consumption is positively associated with either gastric or colorectal cancer.” (World Health Organization)
On April 1, 2020, the FDA issued a recall on Zantac. In addition to NDMA concerns, “the agency has determined that the impurity in some ranitidine products increases over time and when stored at higher than room temperatures may result in consumer exposure to unacceptable levels of this impurity.”
If you have been diagnosed with cancer after taking Zantac regularly, please contact us immediately by calling 504-523-2434 or filling out an online Contact Form for a FREE Consultation.
Unacceptable levels of carcinogens in Zantac | Zantac Lawsuits
Online pharmacy Valisure notified the Food and Drug Administration (FDA) in June 2019 that routine testing done in their labs had linked Zantac to a carcinogen chemical (a substance or agent causing cancer) called N-nitrosodimethylamine (NDMA). Following the notification and a petition for recall filed by Valisure, FDA’s own testing found unacceptable levels of NDMA in Zantac.
In September 2019, CVS, Walgreens, Rite Aid and Wal Mart suspended the sales of Zantac/Ranitidine products in the US. The next month, the Memorial Sloan Kettering Cancer Center removed Zantac from the list of drugs that it offered to patients. In October, Sanofi finally announced a recall in the US.
If you have regularly taken Zantac and were diagnosed with cancer, you should Contact Us Today by calling 504-523-2434 or by filling out an online Contact Form for a FREE Consultation.
Zantac and Cancer
Ranitidine (brand name: Zantac) is known to form NDMA for decades. However, only after an independent online pharmacy lab tested Zantac in 2019 and warned the FDA about what even the agency called “unacceptable” levels of the carcinogen linked to the drug, the manufacturer issued a recall.
Possible side-effects include:
- Bladder cancer
- Colon and rectal cancer
- Esophageal cancer
- Intestinal cancer
- Kidney cancer
- Liver cancer
- Lung cancer (non-smokers)
- and more
Peiffer Wolf Carr Kane & Conway has represented thousands of clients from across the United States in cases related to pharmaceutical products and medical devices. If you have regularly taken Zantac and were diagnosed with cancer, you should Contact Us Today by calling 504-523-2434 or by filling out an online Contact Form for a FREE Consultation.
Zantac Lawsuits | FREE Consultation
Peiffer Wolf Carr Kane & Conway has represented thousands of clients from across the United States in cases related to pharmaceutical products and medical devices. If you have regularly taken Zantac and were diagnosed with cancer, Contact Us Today by calling 504-523-2434 or by filling out an online Contact Form for a FREE Consultation.
PHARMACEUTICAL & MEDICAL DEVICES IN THE NEWS
Yes. Please call us or use our contact form to request a Free Case Evaluation. We have a national team of attorneys and staff who look forward to speaking with you.
Typically, we represent clients on contingency fee agreements. If we take your case under a contingency fee arrangement, you won’t owe our firm any legal fees unless we are able to recover money for you.
Our contingency fee agreements are usually based on a percentage of the amount we recover for our clients. The contingency fee amount is determined by the type of case, our estimate of how long it will take to resolve your case, and our estimate of the litigation costs we will advance in your case. Each engagement agreement includes the details of the fee arrangement. Questions about our fee agreements are welcomed and encouraged.
In most litigation matters, it is extremely difficult – practically impossible – to predict how long it will take to resolve a particular case. Every case is different, and we will do our best to provide you with an estimate based on your case and our experience with similar cases. Moreover, we will do our best to keep you updated and manage expectations along the way.
We handle cases that change lives. Contact us today for a FREE consultation.






